Our bodies are built out of cells, just like houses are built of bricks. After an earthquake, house repairs would require new bricks to rebuild. The same holds for our bodies. To recover after injury, fight disease and simply stay alive we need new cells every day.
However as time passes, the supplies of new cells grow short and the old cells become weaker. These factors generally contribute to our aging.
Why is this happening? Is there a way to protect our cells and ourselves from aging?
Let us find out.
The origins of new cells
New cells are created by splitting the old cell into halves. It starts with making a copy of a DNA molecule. Since DNA encodes all the guidelines necessary for cell function, proper DNA replication is of utmost importance. Unfortunately for us, this process never works perfectly. Some pieces at the ends are always lost, so the copy turns out a bit shorter than the original.
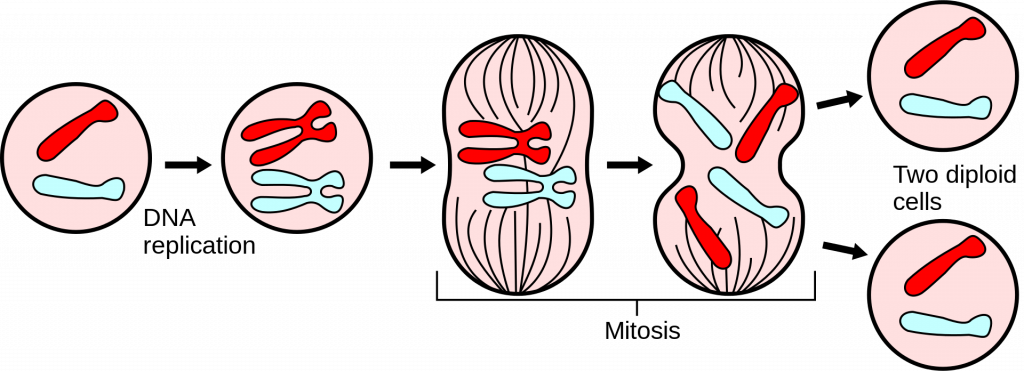
Mitosis – a process of mother cell dividing and giving birth to daughter cells (Source: Wiki)
Telomeres – a nonsense sacrifices
Imagine that you are typing a report for your boss. However, you have a faulty printer which never prints the first and the last pages. What would you do?
If you were to think like a human cell – you would fill those pages with random letters before printing. As a result, you would not care if they are lost as they did not make sense in the first place.
The ”random letters” on the ends of DNA are called telomeres. Telomeres grow shorter and shorter with every cell division, but it does not affect the life of daughter cells, because no important information is lost.
Why do cells grow old then?
Telomeres are not infinite. After many divisions, they vanish – then the cell stops dividing and becomes senescent. As we age, the number of senescent cells grows.
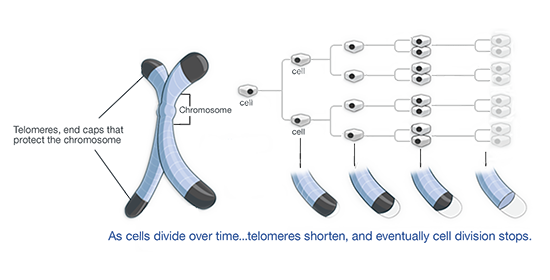
DNA molecules are packed into chromosomes (Source: tasciences.com)
Nevertheless, there is good news: cell senescence can be postponed!
The solution lies in stem cells. What defines a stem cell is the ability to divide an endless number of times and never become senescent. The secret behind this superpower is telomerases – instruments that extend telomeres. Unfortunately, these instruments are unavailable to most of our cells. But maybe if we were able to reactivate telomerases just a bit – we could slow down aging and stay free from the danger of disease. This topic is being researched as we speak and this is an evolutionary precaution to avoid cancer.
Telomerases as anti-aging weapons
The idea is quite simple: if we postpone senescence on a cellular level by elongating telomeres then we postpone the senescence of our body as a whole. This begs the question: how exactly would we arm our cells with telomerases? Which approach would be both efficient and safe?
Three ways of telomerase activation are currently under investigation.
1. Telomerase gene therapy (TGT)
Most therapies first tested in miceWe understand murine longevity better than those to humans. Thus, while TGT had great success in animals – it is not yet available for us. But at this stageg it shows a lot of promise!
What can be achieved with TGT?
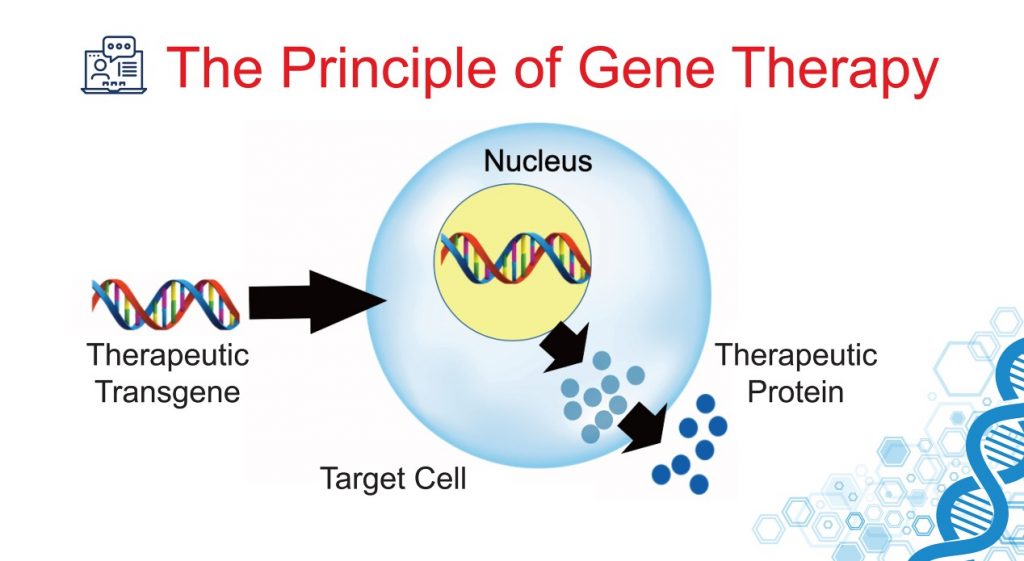
The goal of gene therapy is to add an extra piece of DNA to our cells, which would instruct them to produce telomerases (Source: ISTH)
Mid-adult and aging adult mice were treated with TGT – and it has been a great success. In fact, the lifespan was extended 13-24%. For humans that could mean an extra 10-18 years! The earlier the treatment was applied – the better the result.
On the other hand, when it comes to dreams of eternal life – we all want to extend our youth. Just prolonging senility for a decade is not the point. TGT has the potential to slow down aging and delay age-related diseases.When applied early, TGT postpones senescence, not just lengthens it.
However, if you have already gained aged years – do not despair. To some extent, the aging process can be reversed! TGT was tested on deficient mice, who aged faster and had severe health problems. As a result of treatment, the condition of those mice was significantly improved and their biological clocks went backward for a bit.
2. Drugs
Sometimes the answer lies not in the future, but the past. A component called TA-65 was extracted from a plant used in traditional Chinese medicine for centuries. Several studies have shown that TA-65 treatment results in longer telomeres in humans. However, while TA-65 is now widely available, the scientific community takes the above-mentioned studies with a grain of salt, as some believe they are not trustworthy.
3. Lifestyle changes
There is evidence that our lifestyle also influences telomere length. Factors such as stress, depression, and anxiety contribute to telomere shortage, while special diets and caffeine can support telomerase function. Instead of waiting for a magic pill to arise an extra cup of coffee might be in order.
Risks and limitations of telomerases therapy
Why does it take so long to develop a magic pill?
In 2018 BioViva company declared that they have run the first human test of telomerase gene therapy on one of its employees. They claimed that the treatment indeed extended telomeres and the effect was detectable even three years later. However, this was not a proper clinical trial and thus these results cannot be called trustworthy.
What is the problem of making a proper clinical trial then?
As it was said earlier, natural suppression of telomerases is vital for preventing cancer, as immortality is one of the fundamental features of tumor cells. Overactivation of telomerases is a frequent reason behind this immortality. It is only logical, that the scientific community takes precautions when it comes to the development of telomerase activators.However, we do not have to entirely give up on the idea. So far, TGT treatment did not lead to increased cancer incidence in mice. It just means that we need to have a bit more patience, while scientists invest more effort into working things out. A lot has been achieved already in this field, but there is also a long road ahead. On the other hand, maybe it does not matter how long this road would take and how old we are, when it ends. Once we are there – we can all become young again!
Olovnikov, A. M. A theory of marginotomy. J Theor Biol 41, 181–190 (1973)
Greider, C. W. Telomeres, telomerase and senescence. Bioessays 12, 363–369 (1990)
Tao, L. et al. Caffeine promotes the expression of telomerase reverse transcriptase to regulate cellular senescence and aging. Food Funct (2021) doi:10.1039/d0fo03246h.
Tran, H. T. T., Schreiner, M., Schlotz, N. & Lamy, E. Short-Term Dietary Intervention with Cooked but Not Raw Brassica Leafy Vegetables Increases Telomerase Activity in CD8+ Lymphocytes in a Randomized Human Trial. Nutrients 11, 786 (2019)